What Nerandomilast Means for Future Antifibrotic Development
In recent blogs we have reflected on the promise shown by the orally-delivered preferential PDE4B inhibitor, nerandomilast, now approved by the FDA for the treatment of IPF. After many years of negative clinical data from many programs, and frustration within the field, this molecule now shows promise as a standalone or in combination with nintedanib and, to a lesser extent, pirfenidone in the IPF and PPF populations.
Therapeutic Positioning
The FDA approval and associated label allows nerandomilast to be dosed either as a monotherapy or in combination with the more established nintedanib or pirfenidone. Its label flexibility opens new questions on how best to integrate anti-inflammatory mechanisms into the antifibrotic treatment continuum. It is currently hard to differentiate between the molecules as standalone therapies – they have similar efficacy but different side effect profiles. Given its known anti-inflammatory effects, it is possible that nerandomilast could be an “early” monotherapy where, given the pathophysiology of the disease, it could have the greater impact on disease progression. This may, in turn, depend on much earlier diagnosis of IPF than is currently possible. While further studies, which are currently planned, will help in positioning nerandomilast, it is likely that factors such as cost, previous clinical experience and patient profile will be key considerations in the immediate future.
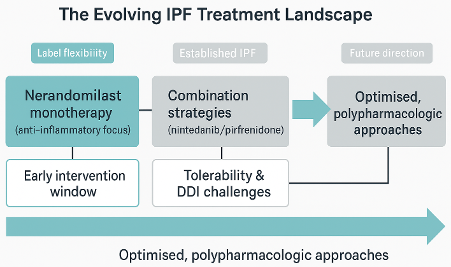
Combination Challenges
Another consideration will be the likelihood of the need to start with or progress to combination therapy. The combination of nintedanib and pirfenidone is poorly tolerated but the Fibroneer trials showed that a combination of nerandomilast with established therapies showed a benefit in key endpoints such as FVC decline. Unfortunately, DDI complicates the combination of nerandomilast with pirfenidone, to the extent that, in the FIBRONEER-IPF trial, efficacy was not observed in patients who received the low dose of nerandomilast with pirfenidone as background antifibrotic treatment. The nerandomilast/nintedanib combination shows increased side effects, particularly diarrhoea. Alongside cost and lifestyle, these complications will undoubtedly be a consideration when the prescribing physician decides on the choice of initial therapeutic.
Implications for Next-Generation Development
The above summary highlights the problem facing drug discovery groups and investors in developing the next generation of anti-fibrotics. Safe and effective poly-pharmacological approaches represent the “holy grail” for a step change in the treatment of IPF. To achieve this, there is a need to identify molecules with complementary pharmacology to the established therapies and, as far as possible, mitigate the risks of any negative drug-drug interactions. These dynamics illustrate why strategic, translational input early in development is essential. The TherapeutAix team has over ten years of experience in identifying, designing and interpreting preclinical studies to address these questions. Please contact us at info@therapeutaix.com if you would like to discuss how we can help with your project.

